Alkynes: Hydrocarbons which have at least one triple bond between carbon atoms are called alkynes. Alkynes with one triple bond have general formula of CnH2n-2.
Structure: Ethyne or acetylene is the simplest member of alkyne class. In ethyne, two carbon atoms share three pairs of electrons. Remaining one electron on each carbon atom is shared with one hydrogen atom.
Question: Draw structural formula for
a. 1-Butyne b. 2-Butyne
Solution
a.
b.General Methods of Preparation of Alkynes
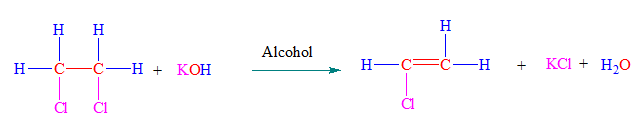
1. By Dehydrohalogenation of vicinal dihalides
A dihalides which have two two halogens at two adjacent carbon atoms is called vicinal dihalide.
When vicinal dihalides is reacted with potassium hydroxide dissolved in alcohol, two molecules of hydrogen halide are removed from adjacent carbon atoms. Removal two molecules of HX gives a triple bond between two carbon atoms.
This reaction completes in two steps.
Tetra halides have four halogens on two adjacent carbon atoms. When tetrahalides is reacted with Zn dust, two molecules of halogen are removed from adjacent carbon atoms. Removal of two molecules of X2 gives a triple bond between to carbon atoms.






Comments
Post a Comment