Question: The bond angles of H2O and NH3 are not 109.50 like that of CH4, although, O- and N atoms are sp3 hybridized.
Answer. The deviation of bond angles of NH3 from tetrahedral angle (109.50) is explained on the basis of repulsion between the lone pair and bond pairs of electrons. As lone pair is closer to the nucleus of the nitrogen, as compared to bond pair. Thus bond angles are decreased. The deviation of bond angles of H2O from tetrahedral angle (109.50) is explained on the basis of repulsion between the two lone pairs and bond pairs of electrons. As lone pairs are closer to the nucleus of the oxygen, as compared to bond pairs. Thus bond angles are decreased.
Question: Which of the following molecules will be polar and non-polar, sketch the structures and justify your answer. (i) CCl4 (ii) SO3
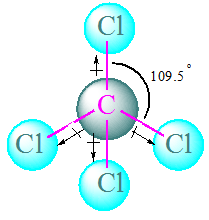
Answer. CCl4 is a non polar molecule. This is due to cancellation of all the dipoles of this molecule due to symmetry. Although, the all C-Cl bond are polar, but molecule is non polar overall. μ = 0 D
μ = 0 D
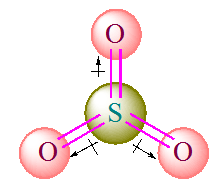
SO3 is a non polar molecule. This is due to cancellation of all the dipoles of this molecule due to symmetry. Although, the all S-O bond are polar, but molecule is non polar overall. μ = 0 D NF3 is a polar molecule. This is due to dipoles of this molecule which do not cancel each other. Hence, SO2 has net dipole moment . SO2 is a polar molecule. This is due to dipoles of this molecule which do not cancel each other. Hence, SO2 has net dipole moment .

Comments
Post a Comment