For example
CH4 (Alkane) CH3-X (Alkyl halide) X = F, Cl, Br and I
Types
Mono, di, tri or poly haloalkane depending upon the number
of halogen atom.
For example
Classification of Alkyl Halides
Alkyl halides can be classified as primary, secondary and Tertiary alkyl halides.
1.
The alkyl halides in which a halogen atom is bonded with primary carbon is called primary alkyl halide. Primary carbon is the carbon that is attached one or no carbon atom.
The alkyl halides in which a halogen atom is bonded with
secondary carbon are called secondary
alkyl halide. Secondary carbon is the carbon that is attached with two carbon
atoms.
For example:
The alkyl halogen in which atom is a bonded with tertiary.
halogen are called tertiary Alkyl bolide.
·Tertiary carbon is Head Carbon that is attached with Three
other carbon atom.
For example:
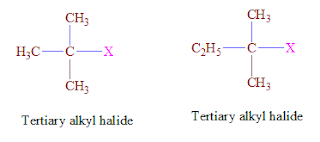
Nomenclature
2- Common LUPAC system of Naming system of Naming
COMMON SYSTEM
IUPAC System of Naming
Physical Properties
Alkyl halides have very high m.p. This is because of bond
polarity of C-X bond (as X halogen is more electronegative than C)
Structure of R-X
Alkyl halide consists of alkyl group which has sp³
hybridized carbon atom bonded with halogen atom.
This C-X band is polar due to move- electronegativity of x.
Preparation of Alkyl halides
(i) From Alcohol
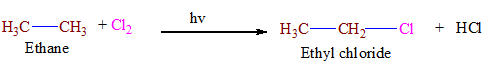
(ii) From Alkane.
Alkyl halide can be obtained from reaction alkane and of sunlight. halogen in the presence
Reactivity of Alkyl Halides
There are two main factor which control the reactivity of alkyl halide.
i. Bond -polarity of C-x bond
ii. Bond energy of C-x bond
i.Bond Polarity
Bond Polarity of R-X is due to move electronegativity of
halogen as compared to carbon
As a result carbon acquires partial positive and halogen
acquires partial negative charge.
So X becomes nucleophile which can be replaced by an another
nucleophile.
On the basis of bond polarity, reactivity of alkyl halide
decreases in following order.
R-F>R-a > R-Br > R-I
ii. Bond energy
Energy required to in Lone energy. break bond of same kind
ide of bond is called bond
Greater the bond energy, lesser will be reactivity of the
beth bond.
Or the basis of bond energy the reactivity of alkyl halide decreases
in following order:
R-I > R-Br >
R-Cl > R-E
Overall The reactivity order of alky) halide is due to bond
energy.
R-I > R-Br > R-Cl > R-F
Definitions
1. Substrate molecule
The alkyl halide molecule on which a nucleophile attacks during
Nucleophilic substitution reaction.
Example:
2. Nucleophile
A specie having lone pair of electrons which attacks on an electrophilic
carbon is called nucleophile.
Example: OH-, C₂H5O-, HS-,
SCN-, H₂O, NH₂- Cl-,
Br-, NH₂-
3. Leaving Groups
A leaving group also nucleophile that is Leaves the
Substrate. Halogen of an alkyl halide leaving group is called leaving group. Example
|
|
Nucleophilic Substitution Reaction (SN)
Definition:
Those chemical reactions where halogen of alkyl halide is substituted or replaced by an attacking nucleophile is called nucleophilic substitution reaction.
During SN two events take place.
i. Formation σ-bond between carbon and
attacking Nucleophile.
ii. Breaking of O-bond between Carbon and
halogen
Mechanism
There are two types of mechanism in SN reaction.
i. Unimolecular nucleophillc substitution
reaction.
ii.
iiBim olecular nucleophillc substitution reaction.
i. Unimolecular nucleophillc substitution reaction (SN1)
The SN reaction in which first bond breaking (between carbon
and halogen) and then bond formation (between carbon and nucleophile) takes
place simultaneously is called unimolecular nucleophilic substitution reaction.
Step 1:
Step 2:
Characteristics of SN1 reaction
1. It is unimolecular reaction.
2. It is two step reaction.
3. First step is slow and it involves ionization
of alkyl halide. It is rate determining step.
4. During first step, carbocation is formed and
carbon bonded with X changes from Sp³ to Sp².
5. Second step is fast and it involves bond
formation between carbon and nucleophile.
6. During second step, carbon of carbocation
changes from sp2 to sp³to sp³.
7. It is favored by Polar Solvent.
8. Tertiary alkyl halide gives this reaction.
9. It gives product with 50% inversion and 50%
retention în configuration.
10. Rate = k[R-x].
Kinetic Evidence of SN1 reaction
Experiments show that the rate of an SN1 reaction
depends upon the concentration of alkyl halide. However change in concentration
of nucleophile does not affect the rate of reaction. Rate = k [R-X]¹. It is a first order reaction
that proves SN1mechanism.
Stereo Chemical evidence of SN1 reaction
Since during reaction, carbon atom of carbonation is Sp²
hybridized and it has unhybridized p orbital. The nucleophile can attack on P orbital
either from right side or from left side.
So it gives a racemic mixture (50% inversion and 50% retention
in configuration).
SN2 (Bimolecular nucleophilic substitution reaction).
The SN reaction in which bond breaking (between carbon and
halogen) and bond formation (carbon and nucleophile) take place simultaneously
is called SN2.
Characteristics of SN2
1. It is bimolecular reaction.
2. It is one step reaction that is rate determining step.
3. In transition state, carbon bonded to halogen atom becomes sp2 hybridized. Both attacking nucleophile and leaving are partially bonded with p orbital in opposite directions.
4. It is favored by non-polar Solvent.
5. Usually primary alkyl halide gives this reaction.
6. It gives product with 100% inversion in configuration.
7. Rate = k[R-x][Nu].
Kinetic evidence of SN2reaction
Experiments show that rate of SN₂ reaction depends upon
conc. of alkyl halide and conc. of nucleophile.
Rate= K(N8] [Alkyl halide]. It is a second order reaction
that proves SN2 mechanism.
Stereochemical evidence of SN2reaction
In SN₂ reaction, nucleophile attacks from opposite side of
leaving group (19). 5. it gives product with 100% invension in configuration.
1,2 Elimination Reaction or beta elimination reaction
The chemical reaction in which two -groups (halogen and B-
Hydrogen) are removed from two adjacent
carbon atoms of alkyl halide to form carbon-carbon double band is called 1,2-
elimination reaction, B-elimination reaction.
These are two types of elimination reaction
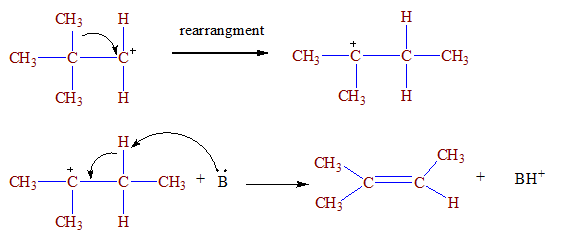
E1-mechanism
The
elimination reaction in which first leaving group is removed to form carbocation and then base removes
beta hydrogen to form carbon –carbon double bond is called E1 mechanism .
First step:
Second step:
Example 2
Characteristics of E1 mechanism
1. It is imolecular elimination reaction.Kinetic Evidence
The experiment show
that rate of beta elimination reaction depends upon the concentration of alkyl
halide and does not depend upon the concentration of base.
Rate = k[R-X]
E2-Mechanism
It is bimolecular elimination reaction in which attack of
base on beta hydrogen and removal of
leaving group with formation of carbocation double take place simultaneously.
Characteristics of E2 mechanism
1. It is a single reaction which is rate determining step.
2. Since two molecule are involves in rate determining step. So,
it bimolecular elimination reaction.
3. Kinetic Evidence
The experiment show that reaction rate of E2 depend upon the
concentration of alkyl and base.
Rate = k [B] [R-X]
Organometallic Compounds (Grignard's Reagents)
Preparation of Grignard's Reagents from Alkyl halides
|
|
|
Alky magnecium halide
Reactivity of Grignard's Reagents
Grignard Reagent show nucleophilic reaction due to partial
negative charge an carbon of alkyl group carbon is more electronegative than
Mg, so it gets partial negative charge.

























Comments
Post a Comment