Electrochemical cells, Electrochemical cells: Definition, construction, working and applications
Question: What are electrochemical cells? Write its different types.
Answer.
Electrochemical Cells
A device that can
convert chemical energy into electrical energy , or uses electrical energy to
drive the chemical reaction taking place in it is called electrochemical cell.
Therefore, electrochemical cells are of two types.
i. Electrochemical cells.
ii. Galvanic cells
Question: What are electrochemical cells? Write its different types.
Answer
Electrolytic cells
An electrochemical
cells in which electrical energy is used to derive the a chemical reaction are
called electrolytic cells.
Construction:
An electrolytic cell
consists of following parts:
a.
A vessel
containing an electrolyte (MX)
b.
Two innert
electrodes
c.
A battery
Working
When two electrodes are
connected with battery, electrons flow from anode to cathode in outer circuit
and the cations move towards cathode and anions towards anode in the solution as
shown in figure. At anode, anions are oxidized by losing electrons while at
cathode, cations are reduced by gaining the electrons.
Applications of Electrolytic cells
Following are uses of electrolytic cells.
1. Down cells are used for preparation of sodium metal along with chlorine gas as by product.
2. Nelson’s cells are used for commercial preparation of sodium hydroxide along with chlorine and hydrogen gases as by products.
3. Electrolytic cells are used for the commercial preparation of calcium, magnesium and many other metals.
4. Electrolytic cells are used for commercial preparation of aluminum metal.
5. Electrolytic cells are also used for purification of copper metal.
6. Electrolytic cells are used for electroplating of tin, silver, nickel etc on steel.
7. Electrolytic cells are also used for prevention of corrosion by preparation of anodized aluminum


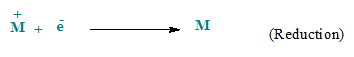
Comments
Post a Comment