Electro-refining
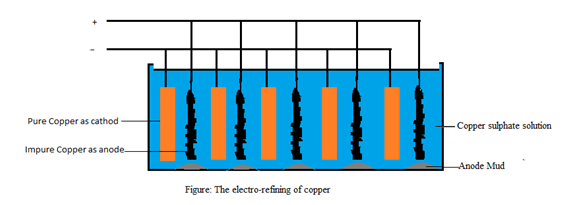
An electro-refining is performed in an electrolytic cell for purification of metals. In this method, impure metal is used as anode and pure metal (same metal as in anode) is used as cathode. For example, electro-refining of copper is carried out in an electrolytic tank containing copper sulphate solution as electrolyte.
On passing electric current through an electrolyte, impure copper dissolves and converts into Cu2+ ions. These Cu2+ ions gain electrons at cathode and form Cu atoms, which get deposited at the cathode. During the process, anode becomes thinner and thinner and cathode becomes large. The impurities like Au and Ag fall off the anode as anode mud.
Following reactions occur during the electro-refining.
Anode Reaction:
Cathode Reaction:
Distillation: Metals with relatively melting points such as As and Hg are purified by distillation.


Good
ReplyDelete