Question: Give three examples of unsaturated hydrocarbons.
Answer.
Ethene Propene EthyneQuestion: Draw electron dot and cross structures for ethene.
Answer.
Question: Draw the structural formulas of an alkane, an alkene and an alkyne containing five carbon atoms.
Answer.
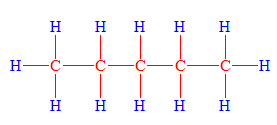
Pentane
1-Pentene
1-Pentyne
Question: How can you differentiate between ethane and ethene?
Answer.
Bromine water is used to differentiate between ethane
and ethane. When bromine water is added to an alkene, the red-brown color
disappears. Disappearance of color is due to addition of Br2 across the
double bond.
On the other hand, when bromine water is added to an alkane,
red-brown does not disappear.
Answer.
A reaction in which water is removed from a molecule is
called dehydration reaction, e.g.
i. Ethene into ethane
ii, Methane into carbon tetrachloride
iii. Ethene into glycol
iv. Ethyl chloride into ethane
v. Ethyl bromide into ethane
Answer.
i.
ii.
iii.
iv.
Ethyl chloride Ethane
v
Question 4: Write a chemical equation to show the preparation of an alkane from an alkene and an alkyne.
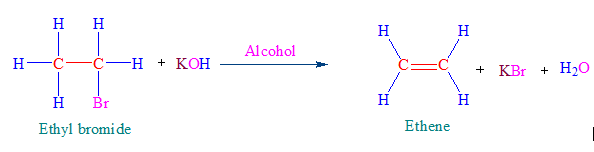
Question 5: Write a chemical equation to show the preparation of ethene from dehydration of an alcohol and dehydrohalogenation of alkyl halides.
Answer.
Question 6: Write a chemical equation to show the preparation of ethyne from dehalogenation of 1,2-dihalide and a tetrahalides.
Answer
Question 7: Write chemical equations showing reaction of KMnO4 with ethene and ethyne.
Answer.
List some industrial uses of ethene and ethyne
Question 8: Explain why a systematic method of naming chemical compounds is necessary.
Answer
Million of organic compounds exist. To understand, recognize and classify these compounds, systematic method of naming these compounds is necessary. An international body '' The international Union of Pure and Applied Chemistry (IUPAC), constantly reviews the rule of naming organic compounds.
Question 9: Draw electron dot and cross structure for
a. Propane b. Propyne c. Propene
Answer













Comments
Post a Comment