Question. What is lattice energy? How does Born- Haber cycle help to calculate the lattice energy of NaCl?
Answer.
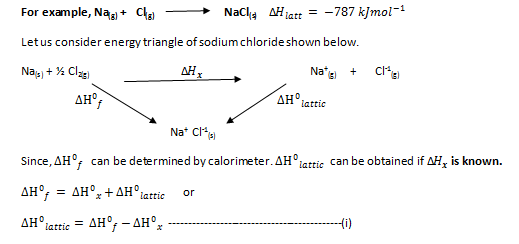
Lattice energy: Amount of energy released when one mole of the ionic compound is formed from gaseous ions.
Now we extend the above triangle to show the various stages involved in finding the ∆ . The complete cycle is called a Born Hyber cycle. This cycle shows all arrows of endothermic processes pointing upward and those for exothermic processes pointing downward.
It is clear from Born-Hyber cycle that
Question. 50 cm3 of 1.0 M HCl is mixed with 50 cm3 of 1.0 M NaOH in a glass calorimeter. The temperature of the resultant mixture increases from 21.0OC to 27.5OC. Assume that calorimeter losses of heat are negligible. Calculate the enthalpy change mol-1 for the reaction. The density of solution to be considered is 1 gm cm-3 and specific heat is 4.18Jg-1k-1.
Answer. Specific heat of water s =4.18 JK-1g-1




Comments
Post a Comment